A Phase III randomized controlled trial comparing the efficacy safety and tolerability of oral dydrogesterone versus micronized vaginal
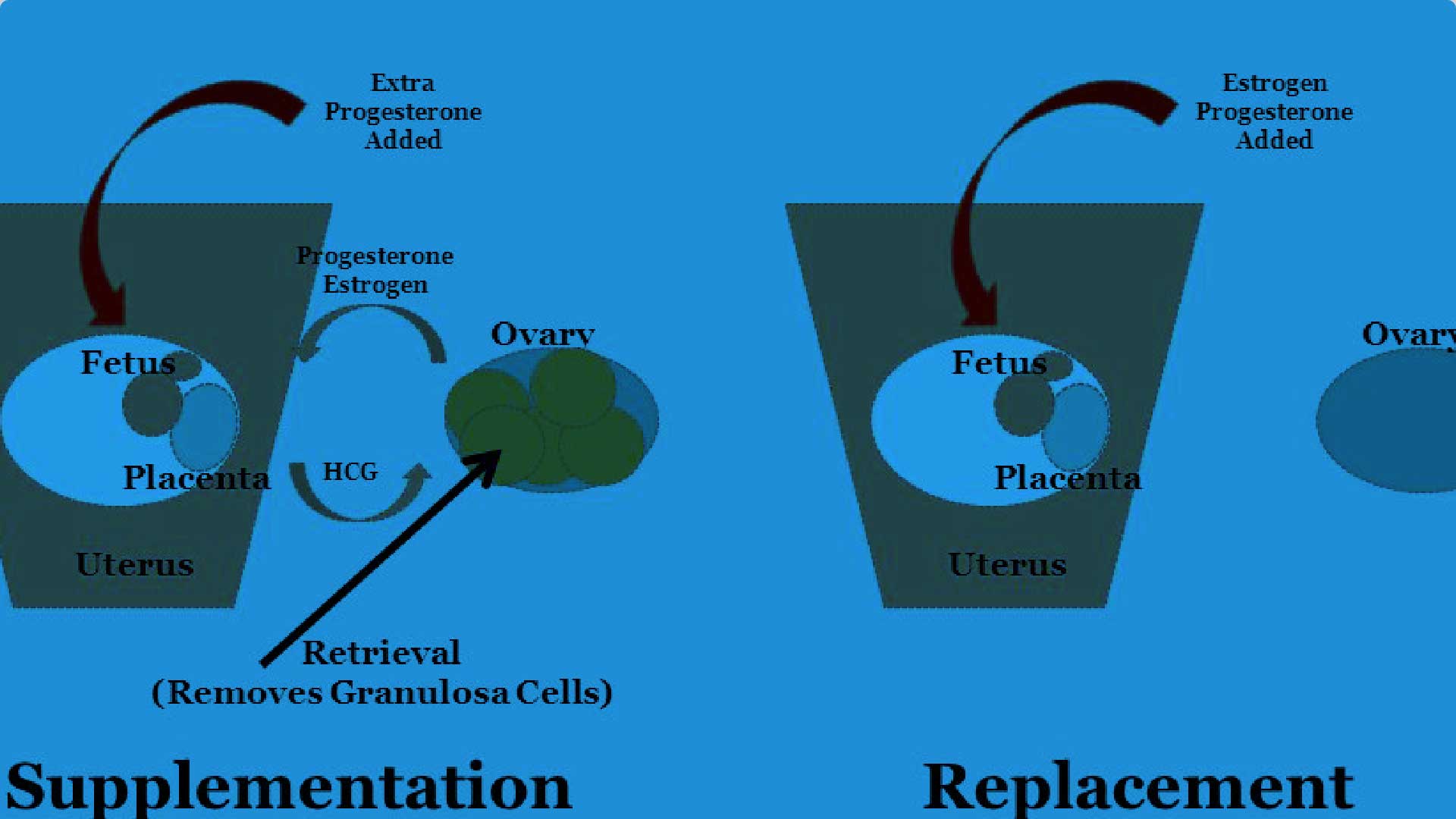
Mar 7, 2021 , Medevista OfficialLuteal phase support, in conjunction with gonadotropin-releasing hormone analogs, is routinely performed during in vitro fertilization (IVF) procedures to overcome luteal phase progesterone deficiency induced
by ovarian stimulation (Practice Committee of the American Society for Reproductive Medicine, 2008; Palomba et al., 2015). Progesterone supplementation or human chorionic gonadotropin (hCG) are commonly administered, although hCG is associated with a higher risk of ovarian hyperstimulation syndrome than progesterone (van der Linden et al., 2015). A systematic review demonstrated that the use of progestogens in IVF was associated with an improvement in the live birth rate (van der Linden et al., 2015). Progesterone can be administered orally, vaginally, rectally, subcutaneously or intramuscularly, although the vaginal route is preferred in the majority of IVF centers globally (Vaisbuch et al., 2012). Indeed, oral administration of progesterone is associated with low bioavailability (due to a substantial firstpass metabolism) and side effects, such as somnolence (Shapiro et al., 2014), while daily intramuscular administration can lead to pain at the injection site, and local abscess (Ghanem and Al-Boghdady, 2012). In contrast, vaginal administration of progesterone can be associated with vaginal irritation, discharge and bleeding (Ghanem and Al-Boghdady, 2012). Therefore, there is an ongoing need for an effective, well tolerated and safe treatment that could also help improve patient satisfaction, convenience and compliance.
0 comments
A Phase III randomized controlled trial comparing the efficacy safety and tolerability of oral dydrogesterone versus micronized vaginal
Mar 7, 2021 , Medevista OfficialLuteal phase support, in conjunction with gonadotropin-releasing hormone analogs, is routinely performed during in vitro fertilization (IVF) procedures to overcome luteal phase progesterone deficiency induced
by ovarian stimulation (Practice Committee of the American Society for Reproductive Medicine, 2008; Palomba et al., 2015). Progesterone supplementation or human chorionic gonadotropin (hCG) are commonly administered, although hCG is associated with a higher risk of ovarian hyperstimulation syndrome than progesterone (van der Linden et al., 2015). A systematic review demonstrated that the use of progestogens in IVF was associated with an improvement in the live birth rate (van der Linden et al., 2015). Progesterone can be administered orally, vaginally, rectally, subcutaneously or intramuscularly, although the vaginal route is preferred in the majority of IVF centers globally (Vaisbuch et al., 2012). Indeed, oral administration of progesterone is associated with low bioavailability (due to a substantial firstpass metabolism) and side effects, such as somnolence (Shapiro et al., 2014), while daily intramuscular administration can lead to pain at the injection site, and local abscess (Ghanem and Al-Boghdady, 2012). In contrast, vaginal administration of progesterone can be associated with vaginal irritation, discharge and bleeding (Ghanem and Al-Boghdady, 2012). Therefore, there is an ongoing need for an effective, well tolerated and safe treatment that could also help improve patient satisfaction, convenience and compliance.
